Cell and gene therapies have the potential to produce dramatic improvements in quality of life and survival for severe and often rare diseases. Currently, however, the cost of these treatments is high and access is sometimes limited. To identify some of these challenges in Europe EURODIS conducted an evaluation of country-specific challenges with respect to four criteria: assessment, affordability, availability and accessibility. These categories are defined as follows
- Assessment relates to the challenges of assessing the benefits and risks of a treatment (e.g., HTA);
- Affordability relates to pricing, funding and affordability;
- Availability relates to non-regulatory issues for making the product available particularly cross-border healthcare and hospital exemptions.
- Accessibility relates to high easy it is for patients to access the treatments and overcome any administrative of other hurdles.
The countries evaluated include Austria, Czechia (Czech Republic), Denmark, England, France, Germany, Italy, Netherlands, Spain, and Sweden. A summary of the results of these investigations is provided in the “Rare Impact” webpage and the results are summarized in the figure below.
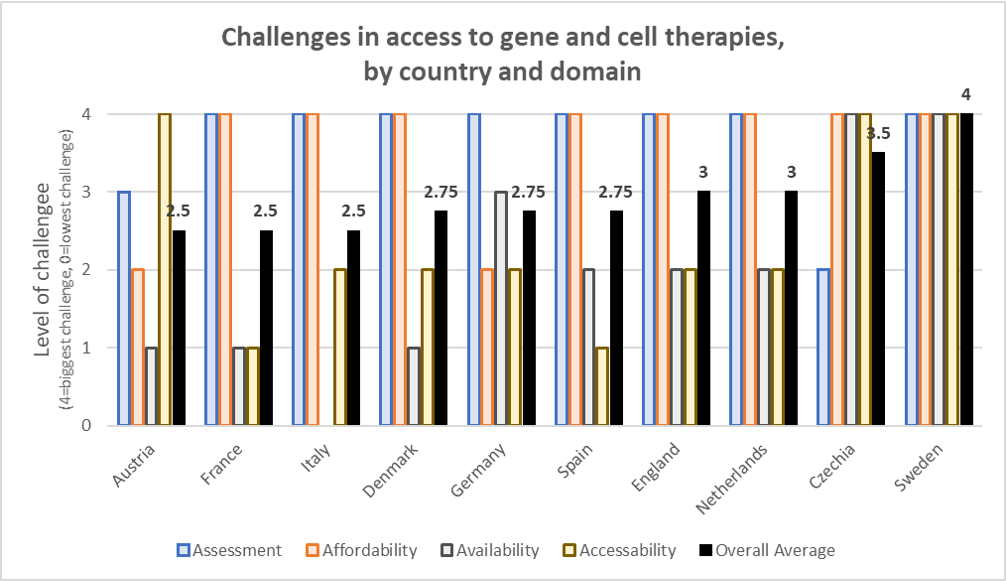
Most of these reports were published in January 2020 and so many be a bit out of date. Nevertheless, some highlights from each country are listed below.
- Austria: Patient access to orphan products in Austria is >90% across all EMA-approved orphan products, a rate similar to that of France. Within the current system, cell and gene therapies are considered hospital products. While these products do not undergo HTA assessment, hospitals have to negotiate cell and gene therapy prices directly with manufacturers. Affordability for patients does not seem to be a major issue. Patients have received access to treatment via cross-border initiatives, but expanding the “Gemeinsam Grenzenlos Gesund” (Unlimited Health Together) cross-border scheme between Austria and Czech Republic could be reconfigured for cell and gene therapies. The report notes that hospital exemption policies are in place and clearly defined. Austria has established the National Centre for Coordination of Rare Diseases (NKSE) to focus on improving access to products for rare diseases.
- Czechia. The Czech Republic spends less on orphan drugs (2.3% of drug spending) compared to peer countries such as Austria (3.8%) or Belgium (6.5%), but Czechia has provided access to some recent CAR-T therapies. Czechia does not have a formal HTA process for cell and gene therapies and manufacturers must negotiate directly with insurers to secure patient access; manufacturers must submit budget impact and CEA models to these insurers. However, insurers focus more on budget impact than long-term value for money. For highly innovative products with uncertain effectiveness, manufacturers can obtain conditional reimbursement for up to 3 years. A compassionate use program (CUP) is also available. “Hospital budgets will initially be responsible for paying for ATMPs, as health care providers have limited budgets for reimbursement of medicinal products (contracted lump sum from health insurers)”. Czechia also may have more limited infrastructure to administer cell and gene therapies and cross-border agreements may be needed.
- Denmark. With respect to HTA assessment, the Danish Medicines Council (DMC) is willing to accept surrogate outcomes, but evidence is graded lower than conventional endpoints. Further, DMC require a minimal clinically important difference (MCID) for use. Affordability issues for cell and gene therapies may exist when hospitals are responsible for delivering specialist treatment. Specialist-prescribed products are funded by hospitals, and the use of such products may be restricted to a single hospital. The availability of specialist funds for cell and gene therapies is unclear, which means that hospitals and health insurers may have to cover these costs. Unlike Czechia, Denmark has better infrastructure to administer cell and gene therapy, but planning across the Scandinavian countries may be helpful to insure sufficient capacity.
- England. NICE has generally given favorable access to cell and gene therapies, perhaps in part due to a desire to grow the country’s life science sector. NICE HTA assessment often depends on highly uncertain extrapolation of short-term trial data on long-term outcomes. There are a number of alternative reimbursement pathways for cell and gene therapies in England including the NICE Single Technology Appraisal (STA), NICE Cancer Drug Fund (CDF), and the NICE Highly Specialised Technology (HST) Programme, with varying cost-effectiveness thresholds ranging from £20,000/QALY to £300,000/QALY. Also, there may be some challenges in adapting NHS work-flows, procedures to the needs of cell and gene therapies, including the ability to incorporate novel payment models. Although NHS allows for cross-border treatment, this is typically approved only as a last resort.
- France. France has been granting access to cell and gene therapies and national-level reimbursement through their standard HTA process with Haute Autorité de Santé (HAS). Treatments that have large medical benefits (ASMR levels I-III) are included in the liste en sus but those with lower medical benefits (ASMR levels IV or V) are included in the liste en sus only if the comparator is only on the list and those with the lowest level of medical benefit (ASMR V or VI) must be funded through a hospital’s budget. Achieving the highest level of medical benefit designation, however, is often difficult is surrogates rather than survival data are used or if the trial is single arm. France has not traditionally used outcomes-based contracting, but that could change as some CAR-T reimbursement included outcomes-based contracts. France planning to have to have a relatively large number of cell and gene therapy manufacturing sites. The importance of CEA is growing in France as treatments with an “…ASMR of I–III and are likely to generate €20m in annual sales in the first two years on the market must submit a cost-effectiveness model to CEESP [Commission d’ évaluation économique et de santé publique]”
- Germany. According to Rare Impact, cell and gene therapies have generally been assessed positively in Germany, patients have received good access to these treatments, and affordability has not been a major impediment to patient access. One key challenge to cell and gene therapy access in Germany is the lack of flexibility in how clinical trial evidence is presented. The Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG) requires a large body of evidence to support the extrapolation of any survival gains based on surrogate trial endpoints and single arm studies are rarely valued highly. “The Arzneimittelmarkt-Neuordnungsgesetz (AMNOG; English translation: Pharmaceuticals Market Reorganisation Act) process is highly structured and emphasizes methodological consistency and rigour. There is relatively little flexibility to account for the particular characteristics of …cell and gene therapies]…that place constraints on trial design and evidence availability at launch… Under new legislation the Gemeinsamer Bundesausschuss (G-BA; English translation: Federal Joint Committee) can now request additional data collection for orphan products with conditional approval.” Outcomes-based contract based on patient survival have already been implemented for CAR-T therapies. One challenge is that cell and gene therapies may be less attractive for German sick funds as patient may switch insurers. German treatments can travel across borders for treatment but this is reviewed on a case by case basis. Although patient access is generally good, there is a risk for additional delays if additional assessment requirements are made companion diagnostic or novel procedure needed alongside the cell and gene therapy.
- Italy. 3 of the first 4 cell and gene therapies to receive marketing authorization were developed in Italy. Italy is the world leader in engaging in outcomes-based arrangement and two CAR-T therapies have been reimbursed using a “payment at result” model. A treatment’s added value depends on: (i) disease severity/unmet therapeutic need, (ii) added therapeutic value and quality of the evidence, and (iii) clinical trials methodology. If innovative status is granted based on value added, this facilitate access at the national level by the Sistema Sanitario Nazionale (SSN) as well as on the regional levels by regional HTA committees; if innovation status is not granted, the Agenzia italiana del farmaco (AIFA) Pricing Committee will ask the manufacture to provide evidence of economic savings such as cost offsets, and these economic factors may take priority. Budget impact plays an important role within Italy’s pricing and reimbursement (P&R) assessments, but because AIFA is in charge of only pharmaceutical spending–rather than total health care spending–decisions typically do not consider any long-term cost offsets. “Reimbursement is often subject to conditions aimed at balancing access and sustainability such as discounts and managed-entry agreements (MEAs) ” By having both national and regional approvals and negotiations, there are more bureaucratic hurdles for cell and gene therapies to secure reimbursement in the Italian market. Italy does already have a specialist fund for “expensive innovative drugs”, but these funds are limited to €1 billion per year; €500m per year for oncology drugs and €500m for non-oncology drugs.
- Netherlands. While inpatient cell and gene therapies are immediately eligible for reimbursement after regulatory approval, if the expected cost is high (>€40m per year or >€10m/year if the cost is >€50,000/per patient/year ) then the Minister of Health, Welfare and Sport can first place the product in the lock (sluice list). Cell and gene therapies in the lock are subject to a CEA assessment–which generally takes 9 months–and requires that medicines meet a €80K/QALY value threshold as reviewed by Zorginstituut Nederland (ZIN). The ZIN evaluation is based on effectiveness, cost-effectiveness, necessity and feasibility; then the Minestry of Health, Welfare and Sport often tries to negotiate a price reduction with the manufacturer. The Netherlands has engaged in the Beneluxa joint assessment initiative on high-priced medicines, but it is unclear to what degree processes are being synthesized in practice.
- Spain. Spain has the reputation of focusing more on cost-containment and affordability and is prone to delays due to the decentralized nature of its pricing and reimbursement system. For instance, pricing decision in Spain are determined nationally, but reimbursement decisions are based on regional level assessments. Budget impact–often measured over only 3 years–is of high importance in Spain. While the national system focuses on ensuring equal access to high-cost medicines across all autonomous regions, each region conducts its own HTA processes which often put greater emphasis on cost-effectiveness than those at the national level. However, the HTA evaluations often do not accommodate data from trials using surrogate endpoints, synthetic control arms, or indirect treatment comparisons. For gene and cell therapies Spain’s National Strategy for Advanced Therapies has been put in place to improve access to cell and gene therapies. Spain allows for reimbursement of products only available outside of Spain if there is high unmet need, but it is not clear if these rules will apply to cell and gene therapies. Like Italy, requirements to negotiate at national and regional levels may cause delays.
- Sweden. Among the countries examined, access to cell and gene therapies in Sweden had the most issues. CAR-T reimbursement has been problematic and many were not recommended by the New Therapy (NT) Council;. Tandvårds-läkemedelsverket (TLV) conducts value-based pricing assessments, but HTA assessment has been skeptical of short-term trials, single arm trials, and those with single are trials as is more common in cell and gene therapies. TLV’s cost-effectiveness threshold does vary by (i) unmet need, (ii) severity of condition and (iii) if there is a limited budget impact due to small populations The Swedish method of evaluation is idiosyncratic: “regions and the NT Council decide which products that should be assessed on the national level in the joint process and which should be evaluated individually by each region” and regardless budgets are held by regions. Sweden has limited experience with novel payment models, such as outcomes-based contracting. If a product is not funded nationally or in a home region in Sweden, patients can receive access to treatment via cross-border initiatives, but these are typically reviewed on a case-by-case basis. Proximity to cell-and-gene treatment centers may also be problematic, particularly in northern Sweden.
No comments:
Post a Comment